

A patient with visceral leishmaniasis at a hospital in South Sudan. Fighting in the region has left some people vulnerable to the disease.Credit: Karel Prinsloo/MSF
Armed conflict in northern Ethiopia between 2020 and 2022 displaced huge numbers of people, driving some into refugee camps elsewhere in the country and in eastern Sudan. Aid agencies struggled to service the remote locations, and people found themselves in areas rife with visceral leishmaniasis, a parasitic disease also known as kala-azar. Now, with Sudan experiencing conflict and city dwellers fleeing to the countryside, there are concerns that history could be about to repeat itself.
In east Africa, visceral leishmaniasis is caused by a single-celled parasite called Leishmania donovani, which is transmitted through the bites of female sand flies (Phlebotomus spp.). Once inside the body, the protozoan invades immune cells. After a few months, the disease manifests as fever, anaemia and swelling of the spleen and liver. “Visceral leishmaniasis is a killer if not treated,” says Eleni Ayele, a physician at the University of Gondar in northern Ethiopia. And refugees and displaced populations in the region are especially vulnerable.
The sand flies that transmit the disease thrive in acacia forests in the border regions of Ethiopia and Sudan, and sleeping outdoors increases a person’s risk of being bitten. A bite is no guarantee of developing disease. “On average, only one out of ten persons who get the parasite into their body go on to develop the disease,” says Koert Ritmeijer, a medical scientist in Amsterdam with international aid organization Médecins Sans Frontières (MSF, also known as Doctors Without Borders). “The other nine will develop a natural immunity.” However, refugees again find themselves at a disadvantage: malnutrition during times of conflict weakens people’s immune systems, making them more susceptible to developing disease. Overcrowding in camps might also boost transmission.
Part of Nature Outlook: Neglected tropical diseases
The link between conflict and visceral leishmaniasis has been shown time and again. After civil war started in 1983 in what is now South Sudan, an estimated one-third or so of one state’s population of 280,000 died of visceral leishmaniasis over the following decade1. When war erupted again in 2013 in the country, cases of the disease more than tripled in some eastern states. And in Ethiopia, Ayele says that the war led to more people with advanced disease, because people were less likely to go to the hospital.
The latest war in Sudan broke out in April, and some civilians are escaping to rural areas known to be leishmaniasis hot spots. Many researchers fear that they know what is coming. Abhay Satoskar, a parasitologist at the Ohio State University in Columbus, visited a hospital in eastern Sudan last December and saw people with the disease first hand. He witnessed camps with tens of thousands of people fleeing conflict in Ethiopia, including many children who had not been exposed to the disease before. “The camps are in endemic areas and they’re expecting a major outbreak there,” says Satoskar. He says an estimated 90% of local people in some villages have signs of a previous infection.
The need for effective defences against the disease is clear, but these have proved tricky to find. Sand flies are difficult to control — they are smaller than mosquitoes, virtually silent and do not require water to lay their eggs. The disease is also tough to treat. In east Africa, the standard therapy is two injectable drugs daily for 17 days — a hard task under the best of circumstances, and next to impossible in a conflict zone in a low-resource country. The drugs can also be toxic to the heart and other organs, and the injections are painful. Many people with the disease must stay in hospital for up to two months, says Ayele.
The hopes of many, therefore, lie in vaccination. An effective vaccine could prevent disease and tamp down transmission. Decades of work has so far failed to produce a vaccine for leishmanisis. But now, researchers pursuing two very different vaccine strategies are moving towards human trials. Success for either would represent a giant step towards addressing a disease that cruelly hits people who are already in troubled situations.
Beyond the traditional
Satoskar is upbeat about the prospect of vaccinating against leishmaniasis. “Immunology and history tell us that vaccination is possible,” he says. For centuries, Bedouin people in the Middle East rubbed parasite sores from an infected person onto mostly-hidden areas of their children’s skin, such as their buttocks. This practice of deliberate infection — known as leishmanization — gave the children skin lesions that are typical of a form of the disease called cutaneous leishmaniasis. But crucially, once recovered the children would be protected from future infections. “It is almost like a vaccine,” says Satoskar. Facial scarring, which parents feared would blight their child’s marriage prospects, could be avoided, albeit at the expense of marks on less visible parts of the body.
Skin complications can occur after treatment for visceral leishmaniasis.Credit: Ahmed Musa
Although cutaneous forms of the disease, such as that caused by the Leishmania major parasites present in the region, are less often fatal than visceral leishmaniasis, the consequences are still significant. “We call them less severe forms of the disease, but that is not what you are thinking if you ever sit down with infected kids and see the lesions on their skin,” says Nathan Peters, an immunologist at the University of Calgary in Canada. The cutaneous form of the disease also proliferates in conflict zones. In 2012, an outbreak of more than 1,000 cases began in Lebanon among those fleeing violence in Syria. Since then, the disease has surged, with reports among Syrian refugees in Turkey and Jordan.
Attempts to create a vaccine for all types of leishmaniasis that offers the same protection as leishmanization began as far back as the 1940s. These were based on live parasites, but the approach was abandoned after decades of work because it could trigger maladies such as large uncontrolled skin lesions. Vaccinologists then turned to killed-parasite vaccines, and, in the 1980s and 1990s, to combinations of proteins from the parasite. All ended in failure, despite initially promising results in animal studies. The disappointments number in the hundreds.
Most vaccines work by triggering an antibody response to part of a pathogen, and the stamping of this memory onto the B cells that produce antibodies. When the invader enters the body, antibodies are waiting to attach to it and flag it for destruction.
This has worked extremely well for many viruses and for bacteria, but single-celled organisms such as Leishmania and the malaria-causing Plasmodium parasite are trickier customers. They have evolved ways to sustain a chronic infection, manipulating the immune system and hiding inside human cells in which antibodies can’t easily find and kill them. What is needed instead is a T-cell response — T cells can recognize and kill infected cells. Unfortunately, a T-cell response is difficult to induce in humans. Moreover, T cells are much tougher to study and measure than are antibodies.
After decades of research, a vaccine that produces antibodies to fight malaria caused by Plasmodium falciparum received the recommendation of the World Health Organization in October 2021. Other malaria vaccines are now being developed, and are providing inspiration for leishmaniasis researchers.
At the University of Oxford, UK, researchers have been using engineered viruses called adenoviral vectors to train the body’s T cells to eliminate malaria parasites hiding in the body2. The vector works by entering liver cells and forcing them to produce malaria parasite proteins. These proteins are then displayed on the cells’ surface, on which they are spotted by T cells that then destroy the infected cells. A malaria vaccine developed on the basis of such technology aims to provoke a lasting memory of the infection so that T cells can swiftly respond should the real parasite ever make its way into the body.
For some leishmaniasis researchers, advances in vaccines that use adenoviral vectors present an opportunity. Paul Kaye, an immunologist at the University of York, UK, says that he and his team have looked to piggyback on investments in vaccines that use these vectors. Kaye is working to ferry the genetic recipe for two Leishmania proteins inside cells using an adenoviral vector. T cells would then recognize and kill these parasitized immune cells, and establish a memory. “We know that viral-vectored vaccines do a good job of generating the CD8 T cells, which have a role in protection against Leishmania, and CD4 T cells,” says Peters.
In 2017, Kaye and his colleagues reported that the vaccine candidate could be administered safely in people3. A subsequent small trial in Sudan provided some evidence of an immune response in people who were experiencing a complication of visceral leishmaniasis that affects the skin and can arise after treatment4. The vaccine was then given to a larger cohort of around 80 people, but this year’s unrest in Sudan has made completing that study difficult. Faced with this enforced interruption, Kaye’s team is now considering switching to the adenoviral vector that was used in the Oxford–AstraZeneca COVID-19 vaccine, because so much was learnt about this vaccine vehicle during the pandemic. But it’s still unclear whether a viral vector will be able to generate enough memory T cells to stop a leishmaniasis infection before it takes hold.
Live and kicking
Another immunization strategy seeks to more closely match what happens during natural infections. Typically, when a person is bitten by a sand fly, saliva and microbes from the insect draw front-line immune cells to the bite. The parasite then enters these immune cells and sets up a lasting infection. “There is huge evolutionary pressure on the parasite to produce these chronic infections, so that they can persist long enough to be picked up by another sand fly,” says David Sacks, a parasitologist at the US National Institute of Allergy and Infectious Diseases (NIAID) in Bethesda, Maryland.
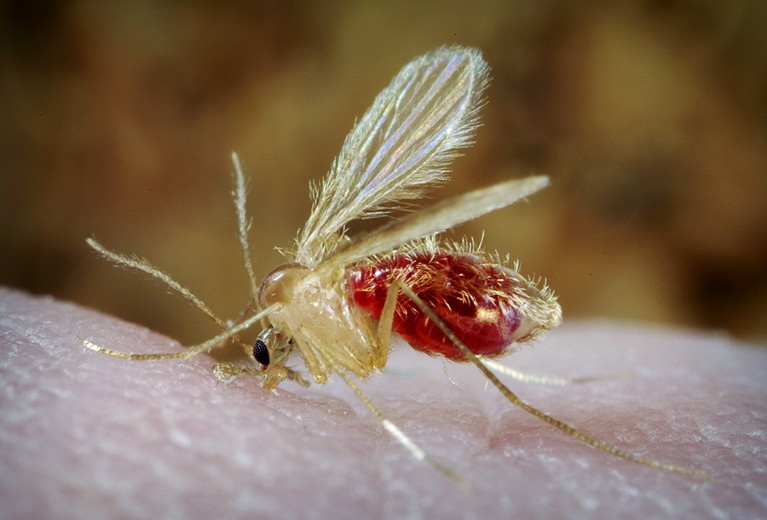
Sand flies (Phlebotomus spp.) are more likely to bite people who are sleeping outdoors.Credit: Scott Camazine/Alam
As Bedouin people have long known, a person who has been infected is immune to secondary infections, and this effect is thought to rely heavily on T cells. Now, a group of researchers in North America wants to emulate the process by returning to the idea of a live vaccine. “Basically, it is leishmanization, except with a safe and defined product,” says Satoskar.
A group led by Hira Nakhasi, a parasitologist and vaccinologist at the US Food and Drug Administration in Silver Spring, Maryland, weakened L. donovani by inactivating a gene. When it was given to mice and hamsters, the weakened parasite could infect cells, but once it was inside it struggled to grow5. “They persist for 7–9 months and then start dying”, without making the animals ill, says Satoskar, who is part of the research team. This seemed to confer long-term protection against future infection in the mice and hamsters.
The team has now switched its attention to the L. major parasites found in the Middle East, which unlike L. donovani typically remain in the skin and should therefore carry less risk. The researchers hope that the species are similar enough that a vaccine based on weakened L. major parasites will provide protection against a broad range of species that cause cutaneous and visceral disease, as well as other species, such as Leishmania braziliensis that causes lesions in mucous membranes of the nose, mouth and throat.
Gennova Biopharmaceuticals in Pune, India, is now manufacturing L. major parasites for use in human studies. “We plan to start a phase I safety trial in the United States and are in discussions with the NIAID,” says Greg Matlashewski, a microbiologist at McGill University in Montreal, Canada, who helped to generate the weakened parasites. The next step would be to vaccinate healthy volunteers and then challenge them with sand flies carrying L. major.
Even injecting genetically-weakened parasites is not a complete facsimile of a natural Leishmania infection. Weakened parasites can persist for many months — long enough to establish an immune memory, the researchers hope — but a natural infection can see parasites remain in the body at low levels for life. “When you do leishmanization, you’re infected forever and this provides beautiful protection from subsequent challenge,” says Peters. Some researchers think that these parasites provide the T cells with constant prodding, and maintain the body’s readiness to quickly suppress any new incursions.
Whether a live vaccine can match the protection afforded by natural infection will become clear only when trials are conducted in endemic regions. However, this will be challenging. For example, heavy rainfall in South Sudan over the past three years has thwarted breeding of sand flies in soils there, suppressing transmission, Ritmeijer says. This means that a trial set up in one place could struggle to return useful data simply because there happened to be few cases that year. The disease also moves around, with people carrying parasites with them into new areas. “In Ethiopia, the disease moves around in clusters. You don’t know where cases will be from one year to the next,” Matlashewski says. As a result, he estimates that a trial to establish efficacy will require at least 5,000 people. Sacks suspects that the figure could be tens of thousands.
In York, Kaye has established a halfway house for vaccine testing, to learn as much as possible about vaccine candidates before establishing field trials to prove their effectiveness. He has grown L. major parasites in his lab, using infected tissue from a young person who had been hiking in Israel’s Negev desert. Around a dozen volunteers in Yorkshire have since allowed female sand flies harbouring these parasites to feed on their arms. After two to four weeks, small lumps appear, which are removed so that the volunteers do not develop leishmaniasis (the parasites present in Israel usually cause only mild illness). Kaye’s group is currently writing up its findings on the early immune response to the parasite — something that is difficult to investigate in a natural setting because signs of infection usually take months to appear, and only then do people visit medical facilities.
The next step for Kaye’s team is to administer its vaccine to volunteers, expose them to infected sand flies and see if it is protective. “If it works, it gives us fresh impetus to test it in real-world settings,” says Kaye. “If it doesn’t work, we’d probably stop.”
A way to go
Adenovirus vaccines and live vaccines are not in competition — each has its advantages. A big positive for adenovirus vaccines is that some countries where leishmaniasis is endemic, such as Brazil and India, now have adenoviral-manufacturing capacity thanks to COVID-19 vaccination efforts. These could be leveraged to produce leishmaniasis vaccines locally. Unlike live vaccines, however, the viral-vector approach has still not been shown to generate enough of a lasting T-cell response to defend against leishmaniasis. “You don’t have the breadth of the immune response you would see with an intact parasite,” says Kaye.
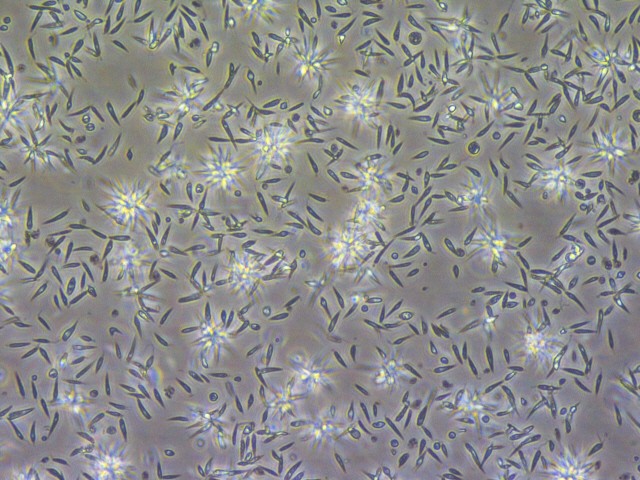
Weakened Leishmania parasites could be used in a vaccine to protect against leishmaniasis.Credit: Gennova Biopharmaceuticals
Evidence from natural infections suggest that live parasites can achieve durable protection, but live vaccines come with considerable logistical challenges. Unlike adenoviral vaccines that can be stored in a fridge, a live-parasite vaccine would have to be stored at −80 °C. Vaccine-delivery infrastructure has improved in endemic areas such as east Africa owing to the COVID-19 pandemic, but getting a live-parasite vaccine into remote rural areas near conflict zones will still be arduous, and it is likely that the associated costs will be greater than for an adenoviral vaccine.
Making vaccines available to the many low-income people at risk is essential to tackling the disease. Kaye estimates that global demand for a vaccine could reach one billion doses over a ten-year period, and that around one-quarter of endemic countries could afford to pay for a vaccine with a cost per dose of around US$5.
Even the most optimistic projections put the approval of a vaccine at 5–7 years away. Each of those years will bring another half a million new cases of visceral leishmaniasis, and at least 50,000 deaths. Many more people will be scarred by cutaneous disease, which infects around half a million people each year. “Children can stop going to school and women are discriminated against,” says the University of Gondar’s Ayele. “It is considered a curse in some parts of society,” she adds.
“We need a vaccine,” says Satoskar. Although it will come too late for the people fleeing war in Sudan right now, it could be the key to finally putting a stop to history repeating itself.
Source link





